
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Pittman RN. Regulation of Tissue Oxygenation. San Rafael (CA): Morgan & Claypool Life Sciences; 2011.

The cardiovascular or circulatory system is designed to ensure the survival of all cells of the body at every moment and it does this by maintaining the immediate chemical environment of each cell in the body (i.e., the interstitial fluid) at a composition appropriate for that cell's normal function. The term “homeostasis” is used to denote the approximate constancy of the internal environment (Claude Bernard, 1866).
First consider the simple hypothetical case of a single spherical cell suspended in a large (>times the cell volume), well-stirred volume of aqueous medium in equilibrium with room air and containing other nutrients. Oxygen availability is often a limiting factor for cell survival, and it is generally supplied to a cell by passive diffusion. As oxygen molecules diffuse into the cell, they are consumed, so that there is a progressive fall in oxygen concentration from the surface of the cell to the lowest concentration which occurs at the center of the cell. For a spherical cell with a typicaldiffusion coefficient for oxygen (≈10 −5 cm 2 /s) and an oxygen consumption of resting skeletal muscle (≈10 −2 ml O2 cm −3 min −1 ), the critical size (radius) which is just adequately supplied with oxygen from the surrounding medium is about 1 mm. Thus, we find that diffusion puts an upper limit on the size of cells in regard to their need for oxygen.
Although diffusion is an efficient transport process over short distances (<100 μm) as seen by the average time required for a molecule to diffuse a distance x (t ≈ x 2 /2D), how can a much larger multicellular organism, such as the human body containing about 100 × 10 12 cells, be adequately supplied with oxygen? For mammals, the bathing medium for cells is water and total body water is about 60% of body weight. For a 70-kg person, total body water is distributed among three compartments with the following approximate volumes: intracellular ≈23 l (33% of body weight); interstitial ≈16 l (22.5% of body weight); and circulating plasma ≈3 l (4.5% of body weight). Cells are bathed in interstitial fluid (ISF), but interstitial fluid volume is only a little more than half the intracellular fluid volume. Thus, ISF cannot be considered a large reservoir of fluid, and its composition is directly influenced by cellular metabolism.
An organism is faced with the following problem: How can the composition of ISF be maintained near its desired value? The solution of this problem is to introduce a circulatory system which continuously refreshes the ISF by putting it in intimate contact with “fresh, reconditioned” fluid (i.e., arterial blood). The circulating blood must be brought close to the cells (<10 μm) since nutrient and metabolic waste exchange takes place by passive diffusion, a transport mechanism which is most efficient over short distances. Thus, the cardiovascular system uses bulk flow (convection) to reduce the effective distance between the pumping action of the heart and the various parts of an organism.
In order for this system to be practical and do its job efficiently, two important conditions must be satisfied: (1) there must be adequate blood flow through the smallest blood vessels, capillaries, which are in contact with the cells comprising a tissue; and (2) the chemical composition of the incoming blood must be controlled to be that which is desired in the ISF. The design and operation of the cardiovascular system fulfill these conditions. Two important functions of the cardiovascular system are to move material (the carrier is blood) and to move heat (tissue metabolism generates heat that must be brought from the body's core to the cutaneous vascular bed at its surface, where it is radiated away from the body).
The systemic circulation and pulmonary circulation are connected in series through the four chambers of the heart, so that all the blood that is pumped from the left ventricle into the systemic organs eventually makes its way back to the right ventricle from where it is pumped into the lungs. The systemic organs (tissues) are connected in parallel, and the following statements are consequences of this parallel architecture: (1) the stroke volume ejected from the left ventricle is divided among the various organs, and a given volume of blood passes through only one organ before entering the venous outflow of the organ; (2) the arterial blood entering each organ has the same composition; (3) the blood pressure at the entrance to each organ is the same; and (4) the blood flow to each organ can be controlled independently (local regulation of blood flow).
The various organs and tissues can be classified as one of two broad types: (1) blood “reconditioners” and (2) “essential” tissues. The main purpose of the blood “reconditioners” is to maintain the composition of the ISF relatively constant under all conditions. In general, flows to these tissues exceed their metabolic needs. Examples of this type of tissue are the lung, which ensures proper exchange of oxygen and carbon dioxide; the kidney, which maintains electrolyte composition and fluid balance; the gut, which oversees nutrient absorption; and the skin, which is involved in temperature regulation. The “essential” tissues are those whose function is critical at all times. The blood flows to these tissues typically match their metabolic needs. Examples of this type of tissue are the heart, which requires a continuous supply of energy to maintain its pumping activity, and the brain, which requires a continuous supply of nutrients and a need for the washout of metabolic products in order to maintain consciousness and carry out its critical functions. One can also add skeletal muscle during exercise to this list, since its energy requirements and needs for washout of metabolic products can be substantial.
A requirement for the circulatory system to carry out its function of bringing blood close to cells so that the exchange of nutrients (e.g., oxygen) and wastes can take place by diffusion is that the blood be able to flow through the complicated networks of blood vessels in the various organs. In order to make a viscous fluid such as blood flow, whether through a single vessel, an organ or the entire systemic circulation, a pressure difference must be applied between the inflow and outflow of the network. The relationship between volumetric flow, Q, and the applied pressure difference, ΔP, is the fluid mechanical equivalent of Ohm's law for electrical circuits and is expressed as Q = ΔP/R, where R is the resistance to the flow of blood. Although the myriad of series and parallel connections of blood vessels in a tissue is quite complicated, each element—a single vessel segment—is simple to deal with.
Poiseuille's law for a viscous fluid quantifies the relationship among the volumetric flow of blood through a blood vessel, modeled as a circular cylindrical tube, the geometric properties of the tube and the flow properties of the blood. Poiseuille's law (1846) is usually expressed as:
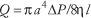
where Q is volumetric flow, the factor π/8 arises from the circular cross-section, a is the radius of tube, l is the length of tube, η is the viscosity of the blood, and ΔP is the pressure difference between the ends of the tube, also called the driving pressure or perfusion pressure. It is noteworthy that the fourth power dependence of flow on radius means that blood flow is quite sensitive to changes in radius, which can vary in the circulatory system as vasomotor tone in vessels controlling flow (i.e., mainly arterioles) changes. It should also be noted that vessel length is generally constant for a given vessel and that viscosity is a property of blood related to the ease with which it can be made to flow. From the relationship among Q, ΔP and R, one finds that R depends on the geometry of the vessel and the viscosity of the blood as
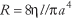
The average velocity of blood through a vessel can also be expressed in terms of the above factors. Conservation of flow leads to the conclusion that volumetric flow is equal to the product of average velocity, v, and the cross-sectional area of the vessel, πa 2 :
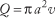

For a Newtonian fluid flowing through a vessel or tube of circular cross-section, the radial dependence of velocity is described as “parabolic” due to the quadratic dependence of velocity on radial position, r:
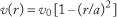
where a is the internal radius of the tube, and v0 is the maximum velocity that occurs on the axis (r = 0); the minimum velocity is zero at the wall (r = a; called the “no slip” condition).
The microcirculation deserves special attention since it is across the walls of these vessels that the exchange of oxygen, among other substances, takes place [82]. Furthermore, the arterioles, also known as the “resistance” vessels, are the primary site for control of blood flow. Thus, the blood vessels of the microcirculation play important roles in both the convective (arterioles) and diffusive (capillaries) transport of oxygen. These blood vessels are classified as arterioles, capillaries and venules and vary in diameter from about 100–200 μm for the largest arterioles and venules down to about 5 μm for capillaries. In terms of their structure, all these vessels possess an inner layer of endothelial cells. In addition, the arterioles have a circumferential layer of vascular smooth muscle with which they can control blood flow and its distribution within organs. Venules typically have thinner layers of smooth muscle.
The primary function of the circulatory system is to exchange substances between blood and tissue, and these exchange processes take place in the microcirculation. The classes of vessels playing a role there are the arterioles (resistance vessels which regulate flow), capillaries (the primary exchange vessels) and venules (exchange and collecting vessels). The amount of flow through the capillaries appears to be regulated to maintain adequate tissue oxygenation. The regulation of blood flow appears to be accomplished by the coordination of several different mechanisms which affect the flow of blood through precapillary vessels.
The transport mechanism of passive diffusion is a rapid and efficient mode of molecular exchange over the small distances (tens of micrometers) between the blood supply (capillaries) and tissue cells. Fick's first law of diffusion (1855) describes the net rate of transfer of a substance from a location of high concentration to one of lower concentration:

where ΔN/Δt is the amount of the substance exchanged per unit time, D is the diffusion coefficient for the substance through the capillary wall, A is the surface area available for diffusion (proportional to the number of blood-perfused capillaries), Δc is the concentration difference across the capillary wall or Δc = c(blood) − c(ISF), Δx is the thickness of the capillary wall (∼1 μm) and P is the permeability of the capillary wall defined as D/Δx.
In regard to the permeability characteristics of the capillary wall, the wall is composed of a single layer of endothelial cells about l μm thick. For lipid-soluble substances (e.g., oxygen), the entire wall surface is available for diffusion. For water-soluble substances (e.g., glucose), there are small aqueous pathways equivalent to cylindrical pores 80 to 90 Å in diameter through which they may pass. Total pore area is about 1/1000 (i.e., 0.1%) of the surface area of a typical capillary. The permeability of the wall to a particular substance depends upon the relative size of the substance and the pore (“restricted” diffusion).
During times of increased activity in a tissue, there is a need for delivery of more nutrients to the active tissue, as well as a need to eliminate accumulated metabolic wastes that result from the increased metabolism of the tissue. The amount of a substance which is exchanged between blood and tissue can be increased by having more of the anatomically present capillaries perfused with blood. This increases the surface area available for exchange and reduces the distance that exchanged molecules must diffuse, both of which increase the efficiency of diffusion. There is some controversy regarding whether it is the number of blood-perfused capillaries that is important or, in the case of oxygen exchange, whether it is the surface area of the capillary wall in contact with moving red blood cells. Under resting, baseline conditions, the equivalent of only a fraction (about 1/3 to 1/2) of the capillaries in a given tissue are being perfused at any given moment. During times of increased demand for nutrients and especially oxygen (e.g., heart and muscle tissue during exercise), more capillary pathways can be opened to flowing red blood cells. Whether a given capillary is open or closed depends on the contractile state of a region of smooth muscle (probably a terminal arteriole) located near the entrance to a capillary [52].
Since the convective supply of oxygen depends directly on blood flow, the regulation of tissue oxygenation depends critically on the regulation of blood flow. The cardiovascular system controls blood flow to individual organs (1) by maintaining the input pressure to each organ within narrow limits by the mechanisms designed to regulate arterial pressure and (2) by allowing each organ to adjust its vascular resistance (R) to blood flow to an appropriate value. The cardiac output (CO) is distributed among the various organs according to their respective resistances so that flow (Q) in an organ is given by:
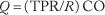
where TPR is total peripheral resistance of the systemic circulation. There are three major mechanisms that control the function of the cardiovascular system: local, neural and humoral. They can work independently of each other, but there are also interactions among them. The local mechanisms are intrinsic to a tissue and will be described in more detail below. The neural mechanisms involve the central nervous system and rely primarily on the release of norepinephrine from the sympathetic nerve endings of the autonomic nervous system. Finally, the humoral mechanisms rely on circulating vasoactive hormones, such as angiotensin II and epinephrine. It is important to recognize that the vasoregulation occurs in the resistance vessels. In the context of the regulation of tissue oxygenation, it is most appropriate to focus on the mechanisms that control blood flow at a local level.
The local mechanisms for regulating blood flow are intrinsic to the various tissues and can operate independently of neurohumoral influences [13,91]. Local regulatory processes allow each tissue in the body some measure of autonomy to satisfy its current and particular requirements in regard to blood flow. Because the various organs and tissues of the body are connected in parallel, the cardiac output can be redistributed among the tissues should their relative need change by altering the resistance (R) to blood flow in the affected tissues.
The site of local regulation of blood flow is the microcirculation, which is composed of a network of blood vessels—arterioles, capillaries and venules—whose functions are regulation oftissueperfusion and exchange of substances between blood and tissue. Although the topology of vascular networks is typically quite complex, as a first approximation, one can think of most networks as a collection of microcirculatory “units” connected in parallel, where each unit is composed of a feeding arteriole, several capillaries arising from the arteriole and a venule which collects the blood after molecular exchange has taken place between it and the interstitial fluid. Because of the parallel structure of the network, which is a collection of these microcirculatory units, it is possible to redistribute blood flow from one region to another within a tissue to accommodate any alterations in local metabolic needs.
Examples of local blood flow control processes are autoregulation, reactive hyperemia and active (or functional) hyperemia. The term “autoregulation” in this context refers to the tendency for organ blood flow to remain constant in the face of local changes in arterial or perfusion pressure. Autoregulation is observed in virtually every vascular bed. It is most pronounced in the brain and kidney and is prominent in the heart, skeletal muscle, intestine and liver. Recall that flow (Q) equals perfusion pressure (ΔP = difference between inflow arterial pressure and outflow venous pressure, Pa − Pv) divided by vascular resistance (R) so that, as ΔP rises through the autoregulatory range (Pa ≈ 80–160 mm Hg in brain and kidney), R must increase to maintain constant flow. Reactive hyperemia refers to the elevated blood flow observed in an organ when flow is restored following a period of circulatory arrest (i.e., occlusion of the blood supply). Hyperemia is literally an excess of blood in a region. The magnitude of the hyperemia is related both to the duration of the occlusion period and to the pre-occlusion blood flow. Active (or functional) hyperemia refers to the increase in blood flow which accompanies an increase in the metabolic activity of an organ or tissue. It has been described in skeletal and cardiac muscle, brain, intestine, stomach, salivary glands, kidney and adipose tissue. The name of the hyperemia depends upon the specific function of the tissue (e.g., contraction hyperemia for muscle or secretory hyperemia for various glands). Each one of these examples of local regulatory processes can be linked to the regulation of tissue oxygenation.
Two major mechanisms have been proposed to account for the local regulatory phenomena described above: the myogenic mechanism and the metabolic mechanism. Although these mechanisms appear to act independently, the expression of each mechanism varies among tissues and some combination of each one is probably operative, depending on the particular intervention, i.e., altered perfusion pressure, flow or tissue activity.
The myogenic mechanism, in essence, states that vascular smooth muscle actively contracts in response to stretch, in an attempt to maintain circumferential wall tension, T, relatively constant in the resistance vessels. The relationship among wall tension (T), intravascular pressure (P), internal radius (a) and vessel wall thickness (w) is given by the law of Laplace (1805) for a cylindrical elastic tube: T = Pa/w. Thus, elastic blood vessels exposed to an increased intravascular pressure will become passively distended. The smooth muscle in the vessel wall responds by active contraction (leading to vasoconstriction) which tends to return wall tension near its baseline value and vascular caliber below its original value. The myogenic mechanism is sometimes referred to as pressure-related control of blood flow.
The metabolic mechanism states that there is a close link between blood flow and tissue metabolism. It has usually been specialized to suggest a link between oxygen supply and demand according to Figure 1.
Schematic diagram of metabolic mechanism for blood flow regulation. Arrows between boxes are each associated with a “+” or “−” sign which indicates that the direction of change in the “downstream or effect” (more. )
Tissue cells continuously utilize ATP as an energy source to maintain cellular function. The two most common ways in which ATP can be produced are by oxidative phosphorylation and glycolysis. Since oxidative phosphorylation is the preferred pathway for most cells to generate ATP, cells have a continuous need for oxygen. In the presence of an adequate supply of oxygen (normoxia), the adenosine diphosphate (ADP) produced from the hydrolysis of ATP is rephosphorylated as part of the process of oxidative phosphorylation, and the contribution of glycolysis to ATP production is negligible. When the supply of oxygen decreases below normal (hypoxia), not all of the ADP is rephosphorylated, and some is degraded further to adenosine monophosphate (AMP) and then to adenosine. Adenosine is a powerful vascular smooth muscle relaxant (i.e., produces vasodilation), and the amount of it produced is tightly linked to the degree of hypoxia. During hypoxia, glycolysis is stimulated, and some of the lost mitochondrial ATP production is made up through this metabolic pathway. The end product of glycolysis, lactic acid, dissociates into hydrogen ion and lactate, both of which also have vasodilator properties. A general principle then is that cells continuously produce metabolic wastes (e.g., adenosine, hydrogen ion, lactate), many of which are vasoactive (usually vasodilator). Metabolite production occurs at a low level, even under normoxic conditions. There appears to be a close linkage between metabolite production and tissue oxygenation, so that metabolite production increases as tissue oxygenation decreases, and vice versa. The main reason responsible for a decrease in metabolite production with increases above baseline in tissue oxygenation is that a small fraction of most tissues are slightly hypoxic at any moment, but temporal variations in the regional distribution of tissue perfusion do not allow situations of chronic hypoxia to develop. Under normal conditions, there is a balance between oxygen supply and demand, but imbalances give rise to local adjustments in blood flow that bring supply back in register with demand.
The following oxygen-linked metabolites have been implicated as potential chemical mediators in the metabolic mechanism of blood flow regulation: adenosine (from ATP hydrolysis: ATP → ADP → AMP → adenosine) and hydrogen and lactate ions (from lactic acid generated by glycolysis). The levels of these metabolites are increased when there is a reduction in oxygen supply relative to demand, leading to tissue hypoxia. The production of more carbon dioxide as a result of increased tissue activity (leading to increased oxidative metabolism) leads to vasodilation through increased H + since carbon dioxide reacts with water to form carbonic acid which rapidly dissociates into H + and bicarbonate ion. Increased release of potassium ion and increased interstitial fluid osmolarity (i.e., production of more osmotically active particles) transiently cause vasodilation under physiological conditions associated with increased tissue activity.
Two of the components in the block diagram above deserve further description. The block labeled “Oxygen Delivery, QO2 = Q [O2]a” refers to the convective flow of oxygen in the arterial blood. Thus, oxygen is delivered by bulk flow of blood (flow = Q) to the exchange vessels (i.e., capillaries) by virtue of its presence in the blood at concentration [O2]a. Hence, increasing blood flow will increase the delivery of oxygen via the blood to the tissues. The block labeled “Metabolite Washout” plays a key role in determining the concentration of vasodilator metabolites in the interstitium, [Vasodilator]ISF. The concept of metabolite washout can be appreciated by considering the movement of the vasodilators produced in tissue cells. They diffuse away from their sites of production, through the interstitial fluid and across the walls of the nearby capillaries (these molecules are generally small enough to pass through the aqueous channels in the capillaries; and most cells have at least one capillary near them). Once the metabolite enters the capillary, it is “washed away” by the blood flowing through the capillary, hence the term “metabolite washout.” It is the summation of metabolite production and metabolite washout that determines the concentration of vasodilators in the ISF in contact with nearby arterioles that control blood flow. Increases in metabolite concentration thus cause vascular smooth muscle relaxation, lowering the resistance to blood flow. Consider exercising skeletal muscle as an example. With the onset of exercise, metabolite production and oxygen requirements both increase. The vasodilator metabolites diffuse away from their sites of production and reach the resistance vasculature through the interstitial fluid. Vasodilation ensues, lowering resistance to blood flow. The resulting increase in blood flow increases the oxygen supply, and finally, a new steady state is achieved in which oxygen supply and demand are matched. This scenario operates for other tissues in which metabolic activity changes.
Several other issues related to the regulation of blood flow, and hence convective oxygen delivery, will be considered here since they have a direct impact on the regulation of tissue oxygenation. The question arises as to whether tissue PO2 closely regulated and whether oxygen plays a direct role in oxygen-linked flow regulation by acting directly on the vascular smooth muscle of resistance vessels. Duling and Berne [18] found that tissue PO2 was regulated within a narrow range, even when the PO2 of a superfusion solution was varied over a relatively wide range of tens of mm Hg. Thus, raising the PO2 of the superfusion solution led to arteriolar constriction, but a relatively constant tissue PO2, suggesting that some components of the tissue and/or the arteriolar wall were sensitive to oxygen and communicated with the arterioles to limit blood flow and oxygen delivery to a desired level. Furthermore, subsequent experiments using a bicarbonate-buffered superfusion solution showed that in the presence of carbon dioxide, tissue PO2 was still regulated but at a higher PO2 than in the absence of carbon dioxide [15]. Duling [15] suggested that there were two possibilities that might explain this finding: (1) the oxygen supply might be regulated by a direct effect on vascular smooth muscle or (2) oxygen acts only through cellular metabolism and that the rate of oxygen delivery to cells, rather than the absolute PO2, would be regulated. Further in vitro [75] and in vivo [16] experiments raised serious doubts that vascular smooth muscle had the requisite sensitivity to oxygen, so that the blood and nearby tissue would need to become severely hypoxic (PO2 of only a few mm Hg) before arteriolar smooth muscle would respond with relaxation. Cytochrome c oxidase, which has a high affinity for oxygen (low KM or P50 of ∼1 mm Hg) and is thus responsive to oxygen levels over a narrow range, was considered to be the oxygen “sensor” in the above studies. Duling [17] later considered that other oxidases and oxygenases, with higher KM's or P50's and which serve other oxygen-linked processes, might take on the role of oxygen sensor.
The novel concept of a mobile oxygen sensor was put forth by Ellsworth and colleagues [24]. The idea is that, when the hemoglobin in RBCs becomes partially deoxygenated, there is a conformational change in the hemoglobin molecule that is transmitted to the RBC membrane and leads to the release of ATP from the RBC. The ATP binds to purinergic receptors on the endothelial cells and results in the relaxation of arteriolar smooth muscle cells, increased blood flow and increased oxygen delivery. A substantial amount of supporting data have accumulated in favor of this means to regulate blood flow, and the idea of a mobile, oxygen-linked sensor that is intimately associated with the carriage of oxygen itself is particularly attractive.
Is tissue PO2 really regulated in the sense of being a controlled variable in a feedback loop which involves a chemical mediator? There are multiple redundant systems which play roles in modulating blood flow [13], and an oxygen-linked system is only one of many. However, the maintenance of tissue oxygenation is such an important feature for survival of the organism that it seems necessary that some mechanisms must exist to ensure an adequate oxygen supply to all cells of the organism. Perhaps there is some overall coordination of events which regulates blood flow to ensure removal of metabolic wastes and delivery of oxygen and nutrients, and leads to an oxygen level consistent with maintaining a balance between energy demand and production.
It has been found that the local vasomotor responses can spread from their point of origin to upstream and downstream sites by electrical conduction through gap junctions between endothelial and vascular smooth muscle cells [2,91]. Since the metabolic responses most closely associated with the regulation of tissue oxygenation will be expressed and sensed initially in the terminal branches of the microvascular network (i.e., capillaries and terminal arterioles), their spread to upstream sites will typically lead to increased blood flow, and hence oxygen supply, through increased vasodilation of arterioles. Thus, the local signals confined to perhaps tens of micrometers can exert their influence over a much wider spatial domain of hundreds to thousands of micrometers, thereby recruiting many vessels in the network to participate in the hyperemia. The sensitivity of the vascular wall to various locally produced vasoactive substances (classic metabolic mechanism), shear stress (flow-induced release of NO from the endothelium) and stretch (myogenic mechanism) appears to vary along the vascular network and from organ to organ. The propagated vasomotor responses thus act to coordinate and integrate the regulation of tissue oxygenation.